2025 Evaluation of MgO solubility in MgF₂ – LiF and MgF₂ – LiF – MF₂ (M = Ca, Ba) at 1053–1203 K
페이지 정보
작성자 관리자 작성일 25-12-10 13:56본문
- 학술지명
- Journal of Magnesium and Alloys
- 개재년월(등록연월)
- 2025-10
- 권(호)
- online
- ISSN/ISBN/e-ISSN
- 2213-9567
- DOI
- 10.1016/j.jma.2025.09.001
[Abstract]
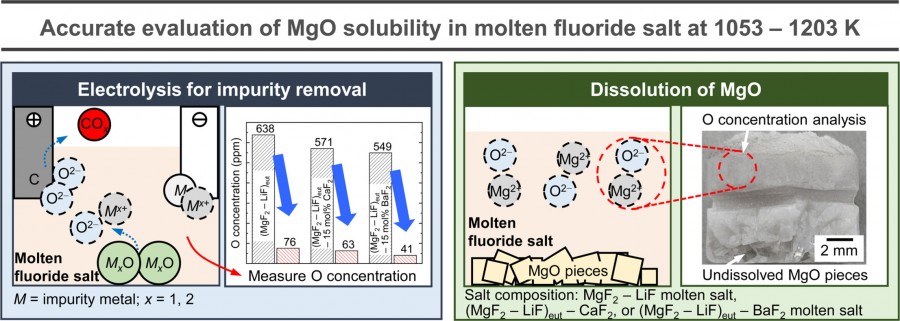
The accurate measurement of magnesium oxide (MgO) solubility in molten fluoride salts is crucial to optimize the electrolytic process for producing high-purity magnesium (Mg) metal from MgO. In this study, the influences of time, temperature, and composition of molten salts such as magnesium fluoride (MgF₂) – lithium fluoride (LiF), MgF₂ – LiF – calcium fluoride (CaF₂), and MgF₂ – LiF – barium fluoride (BaF₂) on the solubility of MgO were investigated. Before the MgO solubility experiments, electrolytic removal of oxygen ions (O²- ) in the molten salts was conducted to decrease the oxygen (O) concentration to below 88 ppm. The results showed that the MgO concentrations in the (MgF₂ – LiF)eut, (MgF₂ – LiF)eut – 15 mol% CaF₂, and (MgF₂ – LiF)eut – 15 mol% BaF₂ molten salts at 1053 K reached saturation to 0.210 mass%, 0.188 mass%, and 0.148 mass%, respectively, after 30 h. Additionally, MgO solubility at 1053 K decreased with increasing concentrations of CaF₂ or BaF₂ in the molten salt. However, the MgO solubility in the molten salts increased with increasing temperature, reaching 0.264 mass% in the (MgF₂ – LiF)eut molten salt at 1203 K. Moreover, increasing the concentration of MgF₂ in the MgF₂ – LiF molten salt increased the MgO solubility at 1103 K. This study provides valuable insights into the MgO solubility in molten fluoride salts used for the electrolytic process using MgO feed for the production of Mg metal.