2025 Investigation of the Carbothermic Reduction of Lithium Sulfate for the Production of Lithium Sulfde
페이지 정보
작성자 관리자 작성일 25-12-09 17:46본문
- 학술지명
- Journal of Sustainable Metallurgy
- 개재년월(등록연월)
- 2025-08
- 권(호)
- online
- ISSN/ISBN/e-ISSN
- 2199-3831
- DOI
- 10.1007/s40831-025-01203-2
[Abstract]
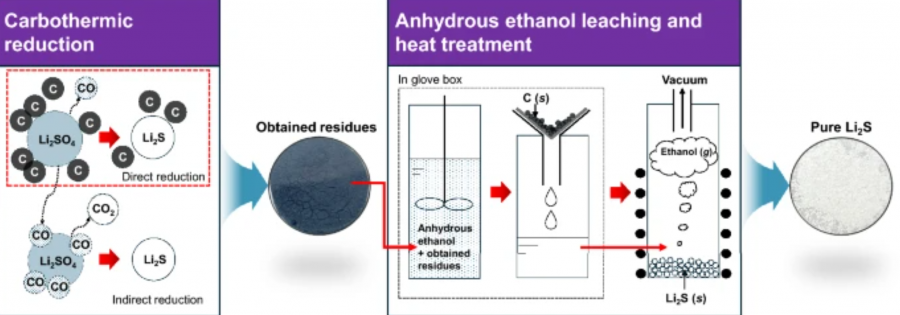
Although lithium sulfde (Li₂S) is a key raw material for solid electrolytes in all-solid-state batteries, its production is costly. To develop an efective production method for high-purity Li₂S, carbothermic reduction of lithium sulfate (Li₂SO₄) was investigated. In this study, the principle of the carbothermic reduction was elucidated using thermodynamic analysis using a chemical potential diagram and experimental results. The reduction of Li₂SO₄ using carbon (C) was performed at 1000–1100 K for 1–10 h under an argon (Ar) gas atmosphere. When the carbothermic reduction was performed at 1100 K for 10 h, a mixture of Li₂S and C was obtained. To produce high-purity Li₂S powder, the residues obtained after the reduction were leached using anhydrous ethanol (EtOH) at 298 K, followed by fltration and heat treatment. The results of this study show the mechanism and feasibility of the production of high-purity of Li₂S from Li₂SO₄ by utilizing carbothermic reduction.