2025 Direct production of TiH₂ powder with 0.21 mass%O from TiO₂ using magnesiothermic reduction in high hydrogen chemical potential
페이지 정보
작성자 관리자 작성일 25-12-09 17:40본문
- 학술지명
- Sustainable Materials and Technologies
- 개재년월(등록연월)
- 2025-07
- 권(호)
- 45 (2025)
- ISSN/ISBN/e-ISSN
- 2214-9929
- DOI
- 10.1016/j.susmat.2025.e01540
[Abstract]
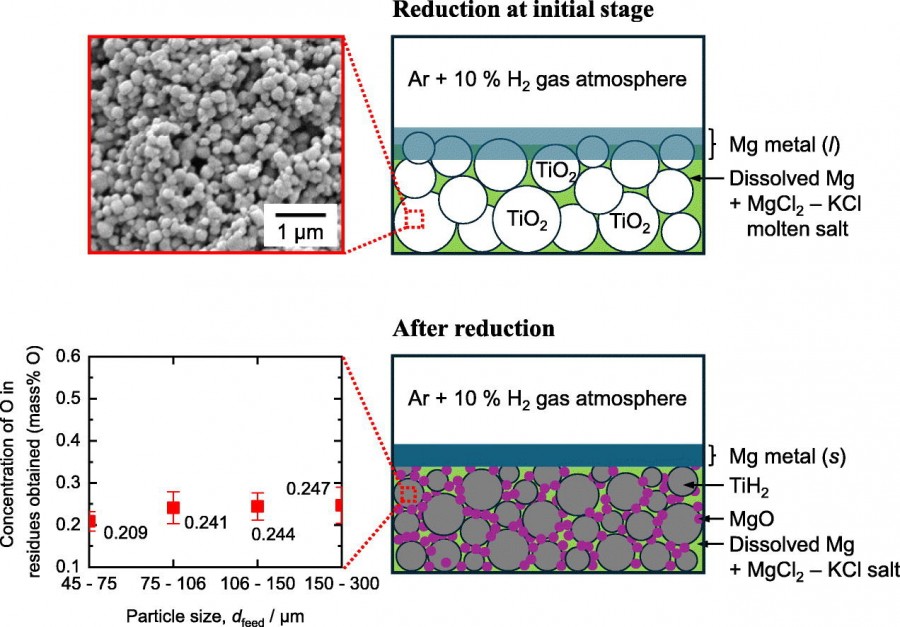
In recent years, reduction followed by deoxidation using magnesium (Mg) in a hydrogen gas (H₂) atmosphere has been suggested as a method to produce titanium (Ti) metal with a low oxygen (O) concentration from titanium dioxide (TiO₂). In this study, a single-step magnesiothermic reduction of TiO₂ in an H₂ mixed gas atmosphere was developed to produce Ti with a low O concentration. In the experiments, the reduction of sintered/unsintered TiO₂ with particle size below 300 μm in molten magnesium chloride (MgCl₂) – potassium chloride (KCl) was conducted at 973 K for 12–48 h in an argon (Ar) – 10 % H₂ gas atmosphere. The influences of microstructure and particle size of TiO₂, the ratio of salt to TiO₂ feed, and reduction time on the O concentration and phases of the obtained residues were investigated. Under certain conditions, TiH₂ powder with 0.209 mass%O was directly produced from TiO₂ powder consisting of micron-scale secondary structures (45–75 μm) formed by the agglomeration of nanosized primary particles. The results of this study demonstrate the feasibility of the suggested single-step magnesiothermic reduction of TiO₂ in high hydrogen chemical potential to produce Ti with a low O concentration.